Methodology
The HepB Calculator
This
calculator is intended to help determine the value of hepatitis B treatment in
specific countries using country-specific information. Users enter in
country-specific costs of disease and treatment and a desired time horizon. The
calculator then simulates health and cost outcomes over that time horizon and
presents overall results under scenarios with and without hepatitis B
treatment. Users can then see how much treatment increases health for a
representative person with hepatitis B. Users can also see how much the
treatment costs over the time horizon (or if it ends up saving costs compared
to no treatment).
More about this calculator on the Lancet: https://www.thelancet.com/journals/langas/article/PIIS2468-1253(19)30223-7/fulltext
The Hep B Calculator evaluates the cost-effectiveness of HBV treatment
from a healthcare payer’s perspective. The tool uses methods compatible with
the WHO CHOICE project1, and as recommended by the Strategic
Information and Modelling Reference Group of the WHO’s Global Hepatitis
Programme2.
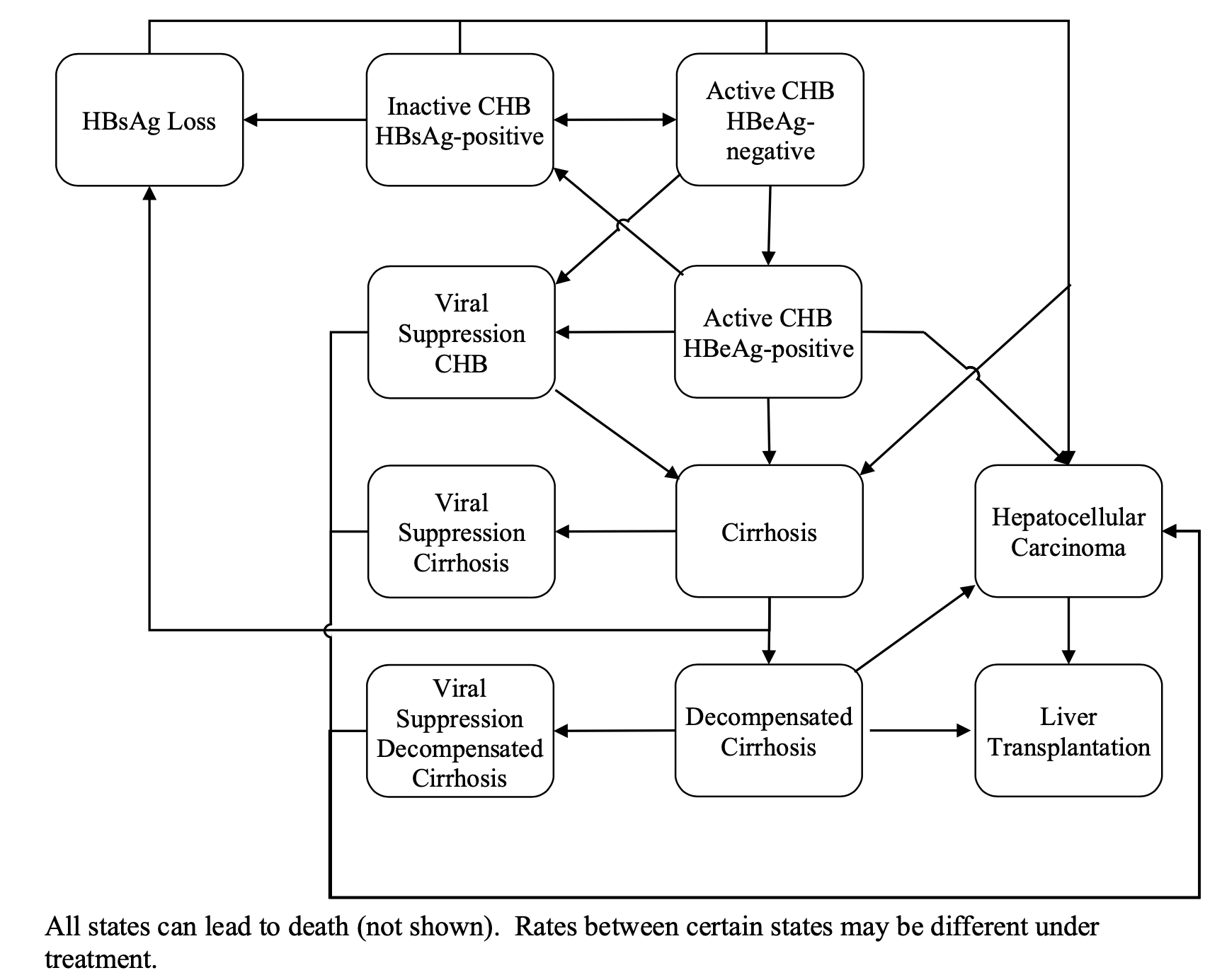
Mathematical model
In the background, the Hep B
Calculator runs a previously-validated mathematical model that simulates the
life course of a cohort of hepatitis B patients, with and without antiviral
therapy3. Treatment-naïve,
chronic HBV, HBeAg-positive or HBeAg-negative
patients eligible for treatment under international treatment guidelines enter
the model either in the cirrhotic or non-cirrhotic health state. From the
initial states patients in the model can transition to other states, response
to treatment (viral suppression), loss of surface antigen, decompensated
cirrhosis, hepatocellular carcinoma, and HBV related death (Figure 1). Age-specific disease
progression3-12 and treatment effectiveness13-17 estimates govern these transitions
in the Markov model. Other causes of death (background mortality) that are not
related to liver disease are included in the model and are based on
country-specific life expectancy from the WHO life tables. The Markov model
calculates using a one-year time step, reported outcomes such as HBV-related
deaths, compensated cirrhosis, decompensated cirrhosis, liver transplants, and
hepatocellular carcinoma. We assigned quality-of-life weights for each
liver-related health state derived from previous studies18 and
aggregated the results into per-person QALYs .
The model compares the
outcomes of two strategies – treatment with antiviral therapy versus no
treatment, and returns, in real-time, the following outcomes for each strategy:
the cumulative life-time incidences of compensated cirrhosis, decompensated
cirrhosis, hepatocellular carcinoma, transplants, and HBV-related death, the
total life-time healthcare costs (including the cost of antiviral treatment and
of downstream events such as liver cancer), and QALYs. In addition, it
calculates the ICERs of antiviral treatment versus no-treatment for different
disease stages, and plots these as graphs to identify time duration after
treatment (in years) when the net cost falls under zero to reach a point where
treatment is cost-saving. The user-interactivity allows for real-time
sensitivity analyses. For example, users can enter different values for local
prices of antiviral therapy, and receive corresponding ICER results that can
help them understand how differences in prices are likely to influence the time
taken for investment in HBV treatment to become cost-effective or cost-saving.
The outcomes of the Calculator can be
printed or saved in an executive-summary style report.
Figure
1: Markov state transition model schematic showing the natural history of
hepatitis B infection.
References:
1.
World Health Organization - Cost effectiveness and strategic
planning (WHO-CHOICE). Retrieved from: http://www.who.int/choice/cost-effectiveness/generalized/en/ (last accesssed: Nov
20, 2018).
2.
World Health Organization – Viral Hepatitis Strategic
Information and Modelling Reference Group: meeting report. Meeting report |
14–16 June 2016, WHO headquarters, Geneva, Switzerland. Available from: http://www.who.int/hepatitis/publications/strategic-information-modelling-meeting/en/
(last accessed: November 23, 2018). Geneva: WHO, 2016.
3.
Toy M, Hutton DW, So S. Population Health
and Economic Impacts of Reaching Chronic Hepatitis B Diagnosis and Treatment
Targets in the United States. Health
Affairs 2018 Jul;37(7):1033-1040.
4.
Chu CM, Liaw YF. HBsAg
seroclearance in asymptomatic carriers of high endemic areas: appreciably high
rates during a long-term follow-up. Hepatology. 2007;45(5):1187-92. Epub
2007/04/28. doi: 10.1002/hep.21612. PubMed PMID: 17465003.
5. Kanwal F, Gralnek IM, Martin P, Dulai GS, Farid M, Spiegel
BM. Treatment alternatives for chronic hepatitis B virus infection: a
cost-effectiveness analysis. Ann Intern Med. 2005;142(10):821-31. PubMed PMID:
15897532.
6. Lin X, Robinson NJ, Thursz M, Rosenberg DM, Weild A, Pimenta
JM, et al. Chronic hepatitis B virus infection in the Asia-Pacific region and
Africa: review of disease progression. J Gastroenterol Hepatol.
2005;20(6):833-43. PubMed PMID: 15946129.
7. Chen YC, Chu CM, Liaw YF. Age-specific prognosis following
spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B. Hepatology.
2010;51(2):435-44. Epub 2009/11/18. doi: 10.1002/hep.23348. PubMed PMID:
19918971.
8. Yuen MF, Wong DK, Fung J, Ip P, But D, Hung I, et al. HBsAg
Seroclearance in chronic hepatitis B in Asian patients: replicative level and
risk of hepatocellular carcinoma. Gastroenterology. 2008;135(4):1192-9. Epub
2008/08/30. doi: 10.1053/j.gastro.2008.07.008. PubMed PMID: 18722377.
9. Chu CM, Liaw YF. Incidence and risk factors of progression to
cirrhosis in inactive carriers of hepatitis B virus. Am J Gastroenterol. 2009;104(7):1693-9.
Epub 2009/05/21. doi: 10.1038/ajg.2009.187. PubMed PMID: 19455130.
10. Chen CJ, Yang HI, Su J, Jen CL, You
SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient
of serum hepatitis B virus DNA level. Jama. 2006;295(1):65-73. PubMed PMID:
16391218.
11. Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, et al.
Carriers of inactive hepatitis B virus are still at risk for hepatocellular
carcinoma and liver-related death. Gastroenterology. 2010;138(5):1747-54. Epub
2010/02/02. doi: 10.1053/j.gastro.2010.01.042. PubMed PMID: 20114048.
12. Fattovich G, Bortolotti F, Donato F. Natural history of
chronic hepatitis B: special emphasis on disease progression and prognostic
factors. J Hepatol. 2008;48:335-52.
13.
Colonno RJ, Rose RE,
Pokornowski K, Baldick CJ, Eggers B, Xu D, et al. Four year assessment of
entecavir resistance in nucleoside naïve and lamivudine refractory patients. J
Hepatol. 2007;46(Suppl. 1):S294.
14. Colonno RJ, Rose R, Baldick CJ, Levine S, Pokornowski K, Yu
CF, et al. Entecavir resistance is rare in nucleoside naive patients with
hepatitis B. Hepatology. 2006;44(6):1656-65. PubMed PMID: 17133475.
15. Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ,
Fang J, et al. Long-term monitoring shows hepatitis B virus resistance to
entecavir in nucleoside-naive patients is rare through 5 years of therapy.
Hepatology. 2009;49(5):1503-14. PubMed PMID: 19280622.
16.
Ke W, Liu L, Zhang C, Ye
X, Gao Y, Zhou S, et al. Comparison of efficacy and safety of tenofovir and
entecavir in chronic hepatitis B virus infection: a systematic review and
meta-analysis. PloS one. 2014;9(6):e98865. Epub 2014/06/07. doi:
10.1371/journal.pone.0098865. PubMed PMID: 24905092; PubMed Central PMCID:
PMC4048232.
17. Gordon SC, Krastev Z, Horban A, Petersen J, Sperl J, Dinh P,
et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with
chronic hepatitis B with high baseline viral load. Hepatology.
2013;58(2):505-13. Epub 2013/02/01. doi: 10.1002/hep.26277. PubMed PMID:
23364953; PubMed Central PMCID: PMC3842114.